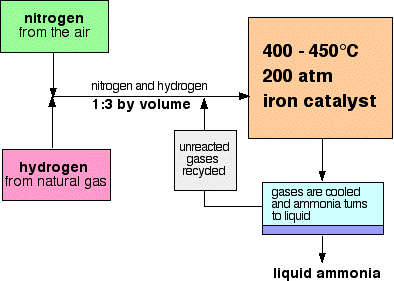
Diagram Source: http://www.chemguide.co.uk/physical/equilibria/haber.html
Need help with the Haber Process? Use the following resources to help you: THIS ANIMATION, the science and details of the process can be FOUND HERE and HERE.
How pressure affects yield:
Because there are four moles of gas on the left (1x N2 and 3x H2), and only two on the right (2x NH3), increasing the pressure has a bigger effect on the left side i.e. it favours the forward reaction [production of ammonia].
How temperature affects yield:
Because the forward reaction (production of ammonia) is exothermic, and the production of nitrogen and hydrogen is endothermic, increasing the temperature causes a decrease in yield of ammonia.






No comments:
Post a Comment